MULT1MODAL POSTOPERATIVE ANALGES1A WITH NONSTEROIDAL ANTI-INFLAMATORY DRUGS AND THE EPIDLJRAL HEMATOMA "MYTH"
Mareos Bolivar. Marcos Bolivar(Jr), Amalia Bolivar, Grisell Var-gas. Hospital de Clinicas Caracas, Acute Pain Service. Anesthesi-ology Dept, Caracas 1015-A, Venezuela
Aim of Investigation: To confirm if the simultaneous use of NSAIDS (nonsteroidal anti-inflammatory drugs) and an epidural catheter produces evidence of epidural hematomas.
Methods: Analgesia records from 2290 patients of the Acute Pain Service at the Hospital de Clinicas Caracas were reviewed. In all patients we placed an epidural catheter for analgesia with bupiva-caine 0.03% and fentanyl at three different concentrations (0.0005%, 0.0003% and 0.0002%). We used in all patients for "multimodal" analgesia during 50 hours, ketorolac in 24% of the patients (initial dose 30-60 mg, plus 30 mg every 8 hours thereafter) and ketoprofen in 76% of the patients (initial dose 100 mg plus 100 mg every 8 hours thereafter. Initially, these NSAIDS were administered intravenously and then changed to oral route as soon as it was reestablished by the treating physician. This technique was used in general surgery, and in several surgical specialties (gynecology, obstetrics, orthopedics, urology, neurosurgery, cardiovascular and thoracic surgery). In all patients blood pressure, pulse, pulse oxymetry, respiratory rate, sedation scale, verbal analogue scale, pruritus, nausea and vomiting, paresthesias and motor blockade were assessed every hour during the first 6 hours, every 2 hours the second 6-hour period, and every 3 hours thereafter.
Results: Observed complications were arterial hypotension 10%, nausea and/or vomiting 11%, urinary retention 7%, sedation 5%, oxygen saturation disturbances 14%, pruritus 17%, paresthesias 10%, motor blockade 5%.
Conclusions: No severe neurological complications were observed, nor signs or symptoms of epidural hematomas were detected.
COMBINING DICLOFENAC WITH PARACETAMOL OR PARACETAMOL+ CODEINE AFTER ORAL SURGERY: A RANDOMISED, DOUBLE BLIND, SINGLE DOSE STUDY
E.K. Breivik. P. Barkvoll*, Dept of Oral Surgery and Oral Medicine, Univ of Oslo, P.O. Box 1109 Blindem, N-0316 Oslo, Norway
Aim of Investigation: To investigate the efficacy ofdiclofenac enterotablets when used with paracetamol or with paraceta-mol+codeine tablets in a single oral dose for acute pain after oral surgery.
Methods: 100 patients in 5 groups experiencing pain intensity above 50 on a 100 mm visual analogue scale following surgical removal of wisdom teeth were included. They received in a randomised and double blind manner a single oral dose of either diclo-fenac 100 mg enterotablets (DI), paracetamol 1 g (P), paracetamol Ig+codeine 60 mg (P+C), diclofenac 100 mg enterotablets + paracetamol Ig (Dl+P), or diclofenac 100 mg enterotablets + paracetamol 1 g+codeine 60 mg (DI+P+C). Pain intensity and pain relief were rated in home-diaries by the patients every 30 minutes for 8 hours. Results were analysed with ANOVA with Duncan's post hoc comparison. Significance was set at P<0.05.
Results: DI+P had superior analgesic effect compared with DI and P alone. DI+P+C were superior to DI alone and P+C alone. These interactions were apparent 2 hours after testdrug intake and persisted until the end of the 8-hour observation period. The incidence of side-effects was not different among the 5 groups.
Conclusion: This single dose study suggests that diclofenac enterotablets increase and prolong the analgesic effect of paracetamol and paracetamol+codeine for acute pain after oral surgery.
SAFETY OF CHRONIC ACETAMINOPHEN ADMINISTRATION. COMPARISON OF TWO DOSING REGIMENS.
H. Ganry'*. A. Peic2', F. Pruvot'', D. Vesque1', J. Dardennes ', N. Schmidely'', J.H. Insuasty'.(SPON: M. Gozariu-Le Bars). 'Laboratoires UPSA / Bnstol-Myers Squibb Company, Rueil-Malmaison, France and ^MS, La Defense Bergeres, 345 av. Georges Clemenceau, Nanterre, France.
Aim of Investigation: To compare the safety profile of chronic treatment with acetaminophen (APAP) 3g/d and 4g/d.
Methods: Retrospective study to identify all patients treated with APAP for chronic rheumatic disease and who presented an adverse event (AE) during treatment. A total of 1.5 million ambulatory patient files, collected in the UK, were reviewed using an indexed medical database. APAP patients were categorized according to their daily APAP dosage: 3g or 4g. In each dosage group were identified patients with hepatic and/or renal AE (HRAE) possibly related to APAP intake. Two analyses were then performed. The two groups of patients (3g or 4g) were compared for incidence of HRAE. Subgroups of patients (3g and 4g) with HRAE were then compared for demographic data, treated disorder, characteristics of acetaminophen intake, etc. The second analysis used Decision Tree Method (DTM). All APAP patients were categorized in two groups: with or without HRAE. DTM determined independent variables best describing differences between profiles of both patients groups.
Results: 7781 patients were identified, 1868 (24%) treated with APAP 3g/d and 5913 (76%) with APAP 4g/d. No difference between dosage groups was observed in all variables including the number of patients with HRAE (16 [0.86%] and 40 [0.68%] in APAP 3g/d and 4g/d respectively). Using the DTM method APAP dosage (3g/d or 4g/d) was never selected to explain HRAE occurrence.
Conclusion: No difference in the safety profile of chronic APAP administration was observed between 3g/d and 4g/d dosage regimens. Chronic APAP daily dosages of3-4g were not correlated with AE occurrence
MULTIPLE 2G DOSES OF PROPACETAMOL, AN INJECTABLE PRODRUG OF ACETAMINOPHEN, DO NOT POTENTIATE LIVER DYSFUNCTION AFTER EXPOSURE TO VOLATILE HALOGENATED ANESTHETIC AGENTS.
M.G. Gerin'. D.Hynes2*, B.Lyons2*, K.Van Holder'*, J.H. In-suasty' - Cappagh Orthopaedic Hospital, Dublin, Ireland and 'Clinical Research Dpt - Laboratoires UPSA / Bristol-Myers Squibb Company, Rueil-Malmaison, France.
Aim of Investigation: To assess the incidence of liver tests (LFT) abnormalities in patients (pts) administered postoperative! y an effective, multiple-dose regimen ofpropacetamol 2g IV or morphine (PCA) after exposure to isoflurane or enflurane during general anesthesia (GA).
Methods: After GA for elective orthopedic surgery, pts were randomly assigned the first postoperative day to receive for 24hrs IV infusions ofpropacetamol 2g, every 4-8 hrs, or morphine for postoperative analgesia. LFTs were performed preoperatively, before treatment, at the end of treatment and 48-96 hrs post-treatment. Laboratory values outside the upper or lower limit of the reference range were considered abnormal. Average values, number ofpts with abnormal values and shift tables were calculated for each laboratory parameter and for groups of parameters; treatments were compared at each period and between periods.
Results: 50 pts were enrolled in each treatment group; in pro-pacetamol group, 25 pts were exposed to 3 doses and 25 to 4 doses, i.e. equivalent to 3 and 4g acetaminophen respectively. Anesthesia and surgery alone induced several LFT' abnormalities. No statistical differences between the two groups were observed in the shift of normality status distribution between pre- and post-treatments. Detailed results will be oresented and discussed.
Conclusions: Potentiation of liver dysfunction induced by enflu-rane or isoflurane was not observed after multiple administrations ofpropacetamol 2g for postoperative analgesia. General biological tolerance, and specifically LFTs, of this propacetamol regimen appears similar to that of an opioid reference treatment.
RECTAL PARACETAMOL (ACETAMINOPHEN) IN ADDITION TO PCA-MORPHINE AFTER ABDOMINAL HYSTERECTOMY.
Oddvar Kvalsvik*. Petter C Borchgrevink* and Ola Dale* (SPON:R. Bell), Dept ofAnaesthesiology and Intensive Care, Trondheim Univ Hospital, Norway.
Aim of the investigation: To evaluate the analgetic effect of rectal paracetamol as an adjunct to morphine after major abdominal surgery.
Methods: 60 patients scheduled for elective benign abdominal hysterectomy were included in a prospective, randomised, double blind, placebocontrolled study to evaluate the effect of rectal paracetamol in conjunction with i.v. morphine. Paracetamol 1000 mg or placebo suppositorium were given four times daily during the 60 hours study period. I.v. morphine was administered via a PCA pump, limited to max 12 mg/hour. Morphine consumption, pain and morphine related adverse effects were recorded. A single analysis was made of corresponding serum concentrations of paracetamol and morphine.
Results: We found a 16% reduction in overall accumulated morphine consumption in the paracetamol group (99,6 vs 83,3 mg) which was not statistically significant (p=0,06). However, paracetamol concentrations were very low, mean 0,03 mmol/1 (0,01-0,06). Patients with higher paracetamol concentration had a lower concomitant PCA-morphine consumption (p=0.03).
Conclusion: We found that the frequently used dosage of rectal paracetamol 1000 mg four times daily is too low. Although there seems to be a significant morphine sparing effect for the patients with highest paracetamol absorption this has to be confirmed in a study using increased paracetamol doses.
COMPARISON OF ANALGESIC EFFICACY AND SAFETY OF PROPACETAMOL 2G AND ORAL ACETAMINOPHEN 1G IN POSTOPERATIVE DENTAL PAIN
P. Lange Mailer''. A. Dillenschneider2', 0. Hiesse-Provost2', J. Insuasty2, S. Sindet-Pedersen'" - (SPON: F. Camborde). 'Dpt of Oral and Maxillofacial Surgery - Royal Dental School - Aarhus Univ, Aarhus, Denmark and clinical Research Dpt - Laboratoires UPSA / Bristol-Myers Squibb Company, Rueil-Malmaison, France
Aim of Investigation: To compare efficacy and safety of a single-dose of i.v. propacetamol 2g (PPA) (injectable prodrug of acetaminophen) administered as an infusion or an injection, oral acetaminophen Ig (APAP) and placebo (P) in postoperative dental pain.
Methods: In this randomized, double-blind, parallel-group study, 175 patients with moderate or severe pain following removal of impacted third molars were randomized. Pain intensity, pain relief (PR), time to perceptible and meaningful PR (double-click stopwatch method), time to remedication and safety were evaluated over a 6-hour postdose period.
|
|
PPA
Inf. n=50 |
PPA
Inj. n=50 |
APAP
n-50 |
P
n=25 |
| TOTPAR (mean) (1) MaxPR (mean) (1) SPID (mean) (1) MaxPID (mean) (1) |
9.35a 2.66a 3.50a 1.34a | 8.78a 2.70a 2.77a 1.28a | 9.70a 2.64a 4.07a 1.38a | 5.02b 1.44b 0.71b 0.64b |
| Nber of patients with onset (2)n (%) (1) Median time to onset (2(min) (1) |
47 (94)a 5b | 47 (94)a 3a | 45 (90)a lie | 14(56)b 13bc |
| Median time to remed. (hrs:min) (1) No longer difference from P for PR (hrs) |
2:51b 3 | 3:00ab 4 | 4:38a 4 | 1:08c
NA |
(1) -. Mediansassosiated with iff. letters
(2) - ; time to perceptible PR when confirmed by a meaningful PR
All active treatments were significantly superior to P for P1D, PR and PRID and at 15, 30 and 45 minutes PPA was significantly superior to APAP. PPA was generally well tolerated. Conclusion: PPA is an effective and fast acting analgesic drug with time to onset of analgesia of around 5 minutes. PPA provides greater relief in the earlier time period than APAP.
A LARGE-SCALE RANDOMIZED CLINICAL TRIAL COMPARING THE TOLERANCE OF IBUPROFEN, PARACETAMOL AND ASPIRIN FOR SHORT-TERM ANALGESIA: METHODOLOGICAL ASPECTS
N. Moore*. E. Van Ganse*, J.-M. Le Pare*, R. Wall*, F. Pelen*, H. Schneid*, M. Farhan*, F. Verriere*, (SPON : F. Hirszowski), Dept of Rheumatology, Hopital A. Pare, 92104 Boulogne-Billancourt, France.
Aim of Investigation: Aspirin, ibuprofen and paracetamol are first-line OTC analgesics. Little is known about the relative risks associated with the use of these drugs in first-line analgesia. It was therefore decided to conduct a randomized controlled blinded study using primarily patient generated data, in France.
Methods: 1300 GPs were to enroll up to 9 patients each, for common pain indications requiring the daily use of analgesics - aspirin or paracetamol (each up to 3g daily) or ibuprofen (up to 1.2g daily) - for at least one day and up to 7 days. Randomization was through central treatment allocation. Patients used a diary to record drug use and adverse events. To ensure compliance with this procedure, the GPs called patients after one and 7 days, but there was no further visit unless medically justified. The primary endpoint was the rate of significant adverse events (serious, severe or moderate events, or events resulting in treatment discontinuation or second GP consultation). All events were reviewed blindly by a Study Safety Committee.
Results: 1147 GPs enrolled a total of 8677 patients. Inclusion data were similar across the study groups: 32% of indications were musculo-skclctal pain, 20% cold or flu, 16% low back pain, 11% sore throat, 10% headache. Mean patient age was 43.5 (SD 14.8), 58.1 % were women. Concerning primary outcome data, 44 patients (0.5%) were unevaluable, leaving 8633 evaluable patients, 8233 (95%) being per-protocol.
Conclusion: This large-scale study of short-term treatment provided high quality patient-generated data, with very few patients lost to follow-up or with missing data.
Acknowledgments: Drs R. Wall and M. Farhan are employees of Boots Healthcare International UK, Drs F. Pelen, H. Schncid, F. Vemere, are employees of Boots Healthcare France.
EFFICACY OF CELECOXIB IN TREATING ACUTE FLARE PAIN IN OSTEOARTHRITIS OF THE KNEE
Roland Moskowitz*, Abraham Sunshine, Emmett Woods*, David Callison*, William Zhao*, Andrew Brugger*. G. Steven Geis* (SPON: Nancy Z. Olson), G.D. Searle Clinical Research, 4901 Searle Pkwy, A-3E, Skokie, IL 60077
Aim of Investigation: To assess the efficacy ofcelecoxib in treating pain of acute flare ofosteoarthritis (OA) of the knee.
Methods: Patient Outcome Questionnaire from the American Pain Society (APS) administered pretreatment and on each of the first seven days of treatment in two identical studies of patients with documented OA flare secondary to discontinuation of anti-inflammatory/analgesic medication. Patients received either placebo (n=145 and 169 in Studies 1 and 2, respectively), celecoxib 100 mg BID (n=143 and 165), celecoxib 200 mg BID (n=141 and 159),ornaproxen 500 mg BID (n= 144 and 169). Results: Two of the five APS measures are shown below:
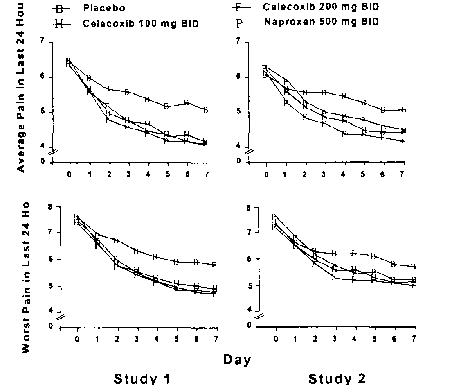
Both celecoxib doses were statistically distinguished from placebo: in Study 1, on all days after Day 1; in Study 2, on all post-baseline days in "Average Pain" (except 100 mg BID on day 2) and on all days after Day 2 in "Worst Pain." Results in the other components of the Questionnaire were similar.
Conclusions: As shown by these replicate trials, celecoxib is efficacious in treating short-term pain in flare ofOA of the knee.
Acknowledgment: Supported by G.D. Searle & Co.
FASTER ONSET OF ANALGES1A WITH EFFERVESCENT ACETAMINOPHEN (EFFERALGAN*) COMPARED TO NON EFFERVESCENT ACETAMINOPHEN. A DOUBLE BLIND PLACEBO CONTROLLED STUDY.
SE Nerholt'*. H Ganry2', L Skoglund'', JH Insuasty2, S Sindet-Pedersen'', J Tagesen'', F Vincent2', PL M0ller''(SPON: A. Cloarec). 'Dpt of Oral and Maxillofacial Surgery- Royal Dental School - Aarhus Univ, Aarhus, Denmark and clinical Research -Laboratoires UPSA / Bristol-Myers Squibb Company, Rueil-Malmaison, France.
Aim of Investigation: To determine and compare onset ofanalgesia and global analgesic efficacy of effervescent (eff) acetaminophen (APAP) (Efferalgan*) and non-effAPAP.
Methods: In a prospective, parallel, double-blind design, patients with moderate to severe pain after third molar extraction, were randomly assigned to receive eff APAP Ig, non-effAPAP Ig, eff placebo (P) and non-effP. Time to perceptible pain relief (PPR) and to meaningful pain relief (MPR) were collected using a stopwatch. Pain intensity (PI-100mm VAS), pain relief (PR-5 point verbal scale) and time to re-medication were assessed over a 6-hour post-dose period
Results
| APAP
efr n=60 |
APAP non-eff+ n=60 |
Placebo effn=62 | Placebo non-effn=60 | |
|
|
||||
| Nber of patients with onset (1) n (%) (2) | 42 (70)a | 42 (70)a | 12(19)b | 9(15)b |
| Median time toonset (1)(min) (2) | 20a | 45b | -
|
-
|
| TOTPAR (mean) (2) | 3.71a | 4.38a | 0.76b | 0.82b |
| MaxPR (mean) (2) | 1.98a | 1.93a | 0.69b | 0.52b |
| SP1D -VAS (mean) (2) | 39.95a | 37.41a | -52.54b | -42.96b |
| MaxPID-VAS (mean) (2) | 24.85a | 22.80a | 4.97b | 4.50b |
| Median time toremed. (hrs:min)® | 2:07a | 2:41a | 1:00b | 1:00b |
(1) - time to PPR when confirmed by a MPR - (2) results associated with different Sellers are significantly different - + 2 x 500 mg
Compared to non eff APAP significant differences favoring eff APAP were observed in PR, PID and PRID during the first 45 minutes after administration.
Conclusion: Eff APAP (Efferalgan*) provides faster pain relief than non-effAPAP. The median time of onset ofanalgesia was considerably shorter, 25 min, as compared with non-effAPAP.
TREATMENT OF ACUTE LOW BACK PAIN WITH NIMESULIDE: RESULTS OF A DOUBLE-BLIND COMPARATIVE TRIAL VERSUS IBUPROFEN
T. Pohjolamen*. A. Jekunen*, L. Autio*, H. Vuorela*, (SPON: M. Kumenius), Jorvi Hospital, Rehabilitation Unit, Turuntie 150, 02740 Espoo, Finland
Aim of Investigation: To define a difference between two drugs on the occurrence of efficacy determined as a relief of lumbosacral back pain or pain related symptoms and to record side-effects occurring during the treatment. In the previous randomized controlled trials it is reported that there is strong evidence that NSAIDs are more effective than a placebo in patients with uncomplicated acute LBP. The efficacy and tolerability of a new COX-2-selective anti-inflammatory drug is unknown.
Methods: A total of 104 patients aged 18-65 years suffering from acute LBP were entered in the study. The patients were randomly allocated to one of two treatment schedules: nimesulide tablets 100 mg twice daily for ten days or ibuprofen 600 mg three times daily for ten days. Functional status was assessed by the Oswestry low-back-pain disability questionnaire at baseline and 24 h, 3 days, 7 days and 10 days after the first tablet. Visual analogue scale was used at baseline, 24 h, 3 days, 7 days and 10 days by modified Million index. Physical examinations, bending measures tests were scheduled at base line, on the 7th and on the 10th day. Details of any side-effects reported were also noted at each visit.
Results: For both study therapies, there was a clear improvement in all measured parameters of pain and back function from the third day of treatment. The changes in the patients' capacity for daily tasks assessed by the Oswestry questionnaire showed improvement for both groups (P< 0.05). At ten days of treatment, a statistical significant difference was found between the two groups (P=0.026) in favor of the nimesulide group. Nimesulide was more effective than ibuprofen in lateral bending measurements with P value of 0.026. In case of modified Schober's test for spinal flexion, improvements were noted in both treatment groups at similar degree. Both nimesulide and ibuprofen gave an early and sustained reduction in mean pain intensity and back stiffness scores as recorded on the VAS scale. No statistical significant difference was observed between the treatment groups. More gastrointestinal side-effects were related in ibuprofen than nimesulide while nimesulide had more general type of side-effects, like tircdness and dizziness. Adverse effects in gastrointestinal tract were met more frequently in the patient group receiving ibuprofen than the group receiving nimesulide but were mild to moderate in severity. The comparison showed a statistically indicative significance (p=0.067) for the difference in occurrence of gastrointestinal adverse effects in patients receiving ibuprofen or nimesulide.
Conclusions: The results of this study confirm that the newer COX-2 selective inhibitor, nimesulide is an effective and well tolerated agent in the general practice management of acute LBP. Serious adverse events were reported in neither treatment group. Some more patients treated with nimesulide reported general type of side-effects and the incidence of gastrointestinal side-effects (in number and severeness) was less with nimesulide than with ibuprofen.
Acknowledgments: Rhone-Poulenc Rorer
EFFICACY OF CELECOXIB IN TREATING ACUTE FLARE PAIN IN OSTEOARTHRITIS OF THE HIP
Abraham Sunshine. Roland Moskowitz*, Emmett Woods*, William Zhao*, Andrew Brugger*, G. Steven Geis*, G.D. Searle Clinical Research, 4901 Searle Pkwy, A-3E, Skokie, IL 60077
Aim of Investigation: To assess the efficacy ofcelecoxib in treating pain of acute flare ofosteoarthritis (OA) of the hip.
Methods: American Pain Society (APS) Patient Outcome Questionnaire administered pretreatment and on each of the first seven days of treatment in a study of patients with documented flare of OA of the hip secondary to discontinuation of anti-inflammatory/analgesic medication. Patients received either placebo (n=211), celecoxib 100 mg BID (n=205), celecoxib 200 mg BID (n=206), ornaproxen 500 mg BID (n=202).
Results: Two of the five APS measures are shown below:
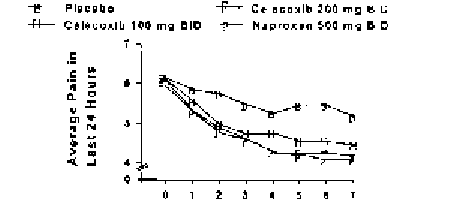
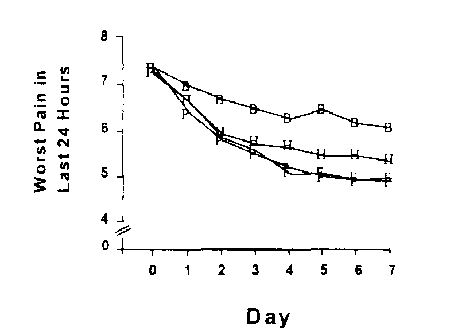
Both celecoxib doses and naproxen were statistically distinguished from placebo on all post-baseline days in both measures. Results in the other components of the Questionnaire were similar. Conclusions: As shown in this trial, celecoxib is efficacious in treating acute pain in flare ofOA of the hip.
Acknowledgment: Supported by G.D. Searle & Co.
ANALGESIC ACTIVITY OF SINGLE IV DOSES OF PARECOXIB, A COX-2 SPECIFIC INHIBITOR, AND TORADOL® IN POSTOPERATIVE DENTAL PAIN
Michael Kuss. Donald Mehlisch, Alicia Bauman*, Doug Baum, Benjamin Schwartz, SCIREX, Austin, TX, G.D. Searle, Skokie, IL
Aim of Investigation: To compare the efficacy of intravenous (IV) dosesofparecoxib(P)(l,2,5, 10, 20, 50 and 100 mg), with Tora-dol® ketorolac (T) (30 mg) and placebo (PBO) in patients with pain following third molar surgery. Methods: Times to perceptible and meaningful pain relief were used to determine time to onset ofanalgesia. Treatments were assessed over 24 hrs using standard scales for pain intensity and relief.
Results: All active treatments were significantly superior to PBO except for PI and P2 for time to onset of pain relief (PBO >24 hrs, T 12min,Pl>24hrs,P2>24hrs,P5 21min, P10 26min, P20 1 Imin, P50 1 Imin and PI 00 9min). Times to perceptible and meaningful pain relief showed similar results. The times to rescue medication for P50 and PI 00 were significantly longer than the other treatments (10:34 H:Min and 13:32 H:Min). P20 and T had similar (8:02 H:Min and 7:53 H:Min) times to rescue medication. Percent of patients not requiring rescue medication over the 24hr period were similar for PBO, T, PI, P2, and P10 (2 - 9.8%) but were higher for the other treatments (P5 19.6%, P20 20%, P50 43.1% and P100 45.1%). Standard efficacy measures (P1D, PR and PRID) yielded similar results.
Conclusions: Parecoxib and Toradol were significantly superior to PBO in onset and duration and other efficacy measures (except PI, P2), and were not different from each other. These data are consistent with data reported previously in an IM dental pain study.
Acknowledgments: This research was sponsored by G.D. Searle & Co.
ONSET AND DURATION OF ANALGESIA OF SINGLE IM DOSES OF PARECOXIB, A COX-2 SPECIFIC INHIBITOR, AND TORADOL* IN POSTOPERATIVE DENTAL PAIN
Donald Mehlisch. Michael Kuss, Alicia Bauman*, Doug Baum, Benjamin Schwartz, SCIREX, Austin, TX, G.D. Searle, Skokie, IL.
Aim of Investigation: To compare the efficacy of intramuscular (IM) doses ofparecoxib (P) (1, 2, 5, 10, and 20 mg), with IM doses of Toradol* ketorolac (T) (30 mg) and placebo (PBO) in patients with pain following third molar surgery.
Methods: Times to perceptible and meaningful pain relief were used to determine time to onset ofanalgesia. Treatments were assessed over 24 hrs using standard scales for pain intensity and pain relief.
Results: All active treatments were significantly superior to PBO except for PI for time to onset of pain relief (PBO >24 hrs, T 14min, P1>24 hrs, P2 1 lOmin, P5 >24hrs, P10 25min and P20 14min). P20 and K were significantly better than the others but were not different from each other. Times to perceptible and meaningful pain relief showed similar results. The times to rescue medication showed the same pattern, PI was not different from PBO and all other groups were. P20 and T had the longest (7:41 H:Min and 8:02 H:Min, respectively) times to rescue medication. These times were not significantly different. Percent of patients not requiring rescue medication following the single dose over the 24hr period were similar for all treatments (8-16%) except for P20 at 34%. Standard efficacy measures (PID, PR and PRID) yielded similar results.
Conclusions: Parecoxib and Toradol were significantly superior to PBO in onset, duration, time to meaningful and perceptible pain relief (except PI), and were not different from each other. Acknowledgments: This research was sponsored by G.D. Searle & Co.
VARIABILITY IN HUMAN RESPONSE TO NSAIDs: INVOLVEMENT OF SEX HORMONES.
John Carmody. Belinda Giles*, Judith Walker, Physiology & Pharmacology, Univ. of New South Wales, Sydney 2052, Australia
Aim: To determine if sex hormones, not gender, per se, are the determinants of females' refractoriness to the analgesic effects of nonsteroidal anti-inflammatory drugs. Methods: Detection and tolerance thresholds for electrically-induced pain in the earlobe were measured in healthy subjects (aged 19 to 57) to study gender differences in the analgesic effects ofibuprofen. Drug (400mg and SOOmg) and placebo were administered once each in a double blind, randomized design. Venous blood was collected for determination of drug concentrations and pharmacokinetic variables. Results were analyzed by ANOVA for repeated measures, using baseline pain as a covariate.
Results: The principal finding was that there are two distinct groups of subjects, one responding to drug treatment (termed Re-sponders) and one that does not (Non-Responders). This outcome accords with results in osteoarthritis and rheumatoid patients as well as experimental inflammation. Interestingly, the proportion of young female subjects in the Non-Responders group was higher than the proportion of young males (38% cf20%). This appears to validate our hypothesis that gender is an issue in interindividual response variability to NSAIDs. There were essentially no pharmacokinetic differences between subject groups, except that females had a greater volume of distribution for ibuprofen (SOOmg). This difference confirms our previous reports and may have been due to gender differences in the partitioning of body water or binding of the drug to plasma proteins. In the females striking differences in analgesic responsiveness were observed at different phases of the menstrual cycle. In both the follicular and menses phases there were high placebo responses in the drug Non-Responders compared to the Responders whereas there was no analgesic response for either subject category in the luteal phase (when levels of progesterone and oestrogen are at their highest).
Conclusions: Sex steroids are likely to be an important influence on NSAID sensitivity. Further study is required of these hormonal influences on response and expectancy theory might prove a profitable approach.
9th WORLD CONGRESS ON PAIN, 1999, Vienna, Austria, p. 447 - 451






