|
NEUROMODULATION DECREASES UTILIZATION OF HEALTH CARE RESOURCES (UHCR): THE CLEVELAND CLINIC
EXPERIENCE
Armin Aeschbach. Nagy Mekhail, Michael Stanton-Hicks; Pain Management Center, Cleveland Clinic
Foundation, Cleveland, 44195, OH, USA
Aim of Investigation: To investigate the role ofneuromodulation implants in decreasing
UHCR for chronic intractable pain patients, specifically the number of physician office and emergency
room visits, hospitalizations, nerve blocks, surgeries, diagnostic imaging studies and the use
of pain medications before and after the implant of a Spinal Cord or Peripheral Nerve Stimulator
(SCS, PNS).
Methods: All 60 patients with age range of 21 to 76 years with SCS/PNS implant between
1991 and 1998 (average duration post implant of 3.4 years [4 months to 8.1 years]) 61% female,
39% male, were contacted by telephone by a disinterested third party and asked to complete a questionnaire.
Results: Primary diagnosis was in 52% Complex Regional Pain Syndrome Type I or II, 38%
failed back surgery syndrome with residual intractable leg pain, 16% Peripheral neuropathic pain,
3% critical limb ischemia. 48 SCS and 9 PNS were implanted, 3 patients had both PNS and SCS. 73%
of patients reported still using their implant on a regular basis. Average reported decreases
in events per year per patient from pre- to post-implant were: Physician visits: 11.4, Emergency
Room visits: 1.40, Hospitalizations: 1.73, Injections/Nerve blocks: 12.27, Surgeries: 0.76, Computer
Imaging studies: 0.76. Medication consumption by the patients who are still using their SCS/PNS:
Narcotics use: discontinued 53%, decreased 20%, maintained 10% and increased 18%. Non-narcotics/analgesics
use: discontinued 30%, decreased 23%, maintained 42% and increased 5%.
Conclusions: Survey results reflect a trend toward an economic benefit associated with
neuromodulation techniques for chronic intractable pain, especially for patients with successful
outcomes. However, uncoordinated treatment and constant care-seeking behavior of chronic pain
patients will negatively influence UHCR.
Acknowledgement: Supported by a grant from Medtronic Inc.
DOES POSTURE AFFECT SPINAL CORD STIMULATION?
K.M. Alo' and T. Cameron2 'Pain and Health Management Center, Houston, TX and Biomedical
Engineering Program, Univ of Texas Southwestern Medical School, Dallas, TX, 75235.
Aim of Investigation: To examine paresthcsia levels produced by spinal cord stimulation
in a population of chronic pain patients implanted with a spinal cord stimulator.
Methods: Electrode leads (Octrode® 2098, ANS, Inc.) were placed percutaneously into the
epidural space under fluoroscopic control (BV29, Phillips, Inc.) at either the cervical or thoracic
vertebral level. All patients were placed in five positions; including sitting, standing, lying
supine, lying right, and lying left. Those with leads implanted at the cervical level were measured
at four additional head positions; these included extension, flexion, right tilt, and left tilt.
At each posture electrical stimulation was applied to the spinal cord. The voltages and pulse
widths necessary to produce initial paresthesia, therapeutic stimulation, and uncomfortable sensations
were recorded. A stimulus frequency of 100 Hz was used for all subjects.
Results: As previously published, we found that the level of stimulation needed for threshold
varied widely between patients and according to posture. The stimulation level need to reach threshold
when leads were implanted in the thoracic level was significantly higher (p < 0.05) when compared
to those implanted in the cervical region. We also found that these postural effects on stimulation
changed with time. Patients who had newly implanted leads (<3 days) required lower stimulation
levels at each posture compared with those implanted permanently (>30 days)(p < 0.05).
Conclusions: Stimulation levels required to produce paresthesia vary between patients,
anatomical locations, posture, and over time. This data further supports the need for multiple
stimulation settings (programs).
Acknowledgments: Supported by ANS, Inc, Alien, Texas.
EPIDURAL SPINAL CORD STIMULATION FOR TREATMENT OF CHRONIC PAIN IN ONE HUNDRED PATIENTS: SOME
PREDICTORS OF SUCCESS.
C. Bonezzi. L. Paulin*, D. Miotti, L. Demartini*, R. Bettaglio*, M. Barbieri and G. Bonctti*,
Pain Management Unit, IRCCS Fondazi-one Maugeri, via Ferrata 8, 27100 Pavia, Italy
Aim of Investigation: The goal of this study was to identify some predicting factors of
success related to spinal cord stimulation (SCS) in chronic pain patients.
Methods: 100 patients with chronic pain were implanted with SCS systems in an eight-year
long period. Indication for SCS included failed back syndrome (21 patients), peripheral neuropathy
(11 patients), complex regional pain syndrome (13 patients), postherpetic neuralgia (2 patients),
peripheral vascular disease (29 patients) and other etiologies of chronic intractable pain (24
patients).
Results: 83 patients received permanent implants 70 of which continue to receive satisfactory
pain relief. Pain due to peripheral vascular disease, peripheral neuropathy, reflex sympathetic
dystrophy and failed back syndrome responded satisfactorily to SCS; in contrast paraplegic pain,
postherpetic neuralgia, cauda equina syndrome, stump pain, phantom limb pain and pain of central
etiology did not respond as well. The best results were obtained in patients in which paresthesia
perfectly covered painful areas. Age, sex and latcrality of pain didn't influence the analgesic
effect of SCS.
Conclusions: Concerning etiopathogenesis of pain we have identified as predicting factors
of success peripheral neuropathic pain without deafferentation and pain due to peripheral vascular
disease. As regard technical aspects we can say that paresthesia covering painful areas is the
main point to be considered.
SPINAL CORD STIMULATION IN FAILED BACK SYNDROME—A COST-BENEFIT ANALYSIS
Keith Budd. The Mommgton Clinic, Bradford BD16 1TW, UK
Aim of Investigation: To investigate the cost-benefit of spinal cord stimulation in "failed
back" syndrome.
Method: In 20 (12> > patients, an in-depth cost analysis of
medical and social treatment was made for 12 months before and after a spinal cord system implant.
Results: 20 patients studied; 12c> (mean age 55.6 yrs), 8 +(mean age 50.5 yrs).
| Costs: 1 yr pre-SCS: |
?1954. |
| 1 yr post-SCS: 1 yr post-SCS + equipt: |
?1250. ?9120.6 |
| Outcome: No problems |
8 |
40% |
| Convert Itref—>Xtrcl |
5 |
25% |
| Electrode shift |
4 |
20% |
| Receiver adjust |
2 |
10% |
| Removed system |
1 |
5%) |
| Work profile post SCS: |
|
| Not working |
12(6+ ) |
| [All off work > 3 yrs pre |
SCSJ |
| Continued work |
4(F+ ) |
| Back to work |
41+ |
Conclusions: In this group ofunselected patients, the series became cost neutral after
5 years by extrapolation. As they were early in the learning curve for SCS, current patients are
cost neutral at 2.8 years after implant.
MOTOR CORTEX STIMULATION FOR CHRONIC NEUROPATHIC PAIN: RESULTS FROM A PROSPECTIVE AUDIT OF
10 CASES AND THE PRELIMINARY FINDINGS FROM AN “N OF L' RANDOMISED, DOUBLE BLIND TRIAL
Dawn Carroll. Tipu Aziz*, Carole Joint*, Henry McQuay, Pain Research Unit, Univ of Oxford, Churchill
Hospital, Oxford and the Dept of Neurological Surgery, Radcliffe Infirmary, Oxford
Aim of Investigation: To determine the effectiveness of motor cortex stimulation (MCS)
in chronic neuropathic pain.
Methods: Eligible patients are implanted with a Medtronic Itrel 2 or 3® device (2-stage
surgical procedure). Resume® quadripolar electrodes are placed extradurally over the motor cortex
strip. In-tra-operative stimulation confirms a motor response in the area of pain. Postoperative
titration to find optimum stimulation parameters is done 4-6 weeks post-operatively. Standard
outcome measures are used to measure pain intensity and pain relief. Volunteered and observed
adverse effects are documented.
Results: 5 of 10 patients treated over a 3 year period responded positively (at least
50% relief) to intermittent MCS. No epileptic seizures were seen in the patients who responded
to stimulation. A seizure was induced in I patient who did not respond during post-operative test
stimulation (9.6 volts). Technical problems including faulty programmers and electrodes were experienced.
Conclusions: 50% of patients treated so far responded positively to MCS. Response was
seen in phantom pain (2), post-stroke pain (2) and post-traumatic neuralgia (1). It is difficult
to predict which patients are likely to respond to MCS, as response does not appear to be condition
specific. Preliminary findings of an 'N of 1 ' randomised double-blind trial will be presented.
The results from the first 2 patients studied suggest that MCS is an effective treatment for pain
and is not just a placebo response. Both patients were able to detect correctly when the stimulator
was switched on or off on 8 of 10 occasions (because of a clear measurable difference in pain).
Updated data from the audit and 'N of 1 ' trial will be presented.
Acknowledgments: No external funding was obtained for this work, which was carried out
within existing NHS resources (Radcliffe Infirmary NHS Trust).
LONG-TERM RESULTS OF CHRONIC MOTOR CORTEX STIMULATION FOR CENTRAL PAIN
Alessandro Dario*. Alberto Dorizzi*, Giovanni Nattero§, Centre of Neurostimulation- Neurosurgical
Dept; Varese Regional Hospital, 21100 Varese, Italy. §Dept of Physiology, Univ of Turin, 10126
Torino, Italy.
Aim of Investigation: Evaluating long-term results of motor cortex stimulation in central
deafferentation pain. Patients and Methods: Four patients (2 males and 2 females; mean age 66
years) underwent an epidurally motor cortex stimulation for central pain refractory to medical
therapy. Two patients had tha-lamic infarction, one had a facial pain due to Wallemberg syndrome
and one developed a facial neuropathic pain secondary to thermocoagulation of the trigeminal ganglion.
All patients were implanted after pain decreased during the propofol test. The results were evaluated
by Visual Analogic Scale (VAS) before surgery and every 3 months after permanent implant (follow-up
ranges from 6 to 41 months, mean 27 months). The complications of the surgical procedure were
also recorded.
Results: At first follow-up the patients with thalamic infarction showed a VAS score improvement
of 60-90%; the last follow-up showed the improvement of the VAS was unchanged. Other patients
showed at first follow-up a VAS improvement of the 50-70%, whereas a progressive pain aggravation
was found, and the last follow-up showed an improvement of the 20-30%. No surgical complications
were found.
Conclusions: These stimulations improve on long-term thalamic pain.
SPINAL CORD STIMULATION VERSUS REPEATED SURGERY FOR CHRONIC PAIN: PRE- AND POSTOPERATIVE
PSYCHOLOGICAL PROFILES IN THE SETTING OF FAILED BACK SURGERY SYNDROME
David H. Kidd, Richard B. North, Dept ofNeurosurgery, Johns Hopkins Univ, 600 North Wolfe St,
Baltimore, Maryland 21287, USA
Aim of Investigation: To determine differences in psychological profiles between patients
with a chief complaint of chronic intractable pain in the setting of Failed Back Surgery Syndrome
(FBSS), with either repeated surgery or Spinal Cord Stimulation (SCS).
Methods: A series of 50 patients presenting with chronic, intractable pain due to FBSS
ofavg. duration of 2.9 yrs, were randomly selected for therapeutic trials of either spinal cord
stimulation, or repeated surgery. They were tested prospectively with three standardized psychological
tests. (MMPI, SCL-90, ABS). These tests were repeated an average of 3 years (1.8-6.8 yrs.) post
operatively. Patients were designated either successes or failures, defined as follow-up of at
least 1 year in duration, 50% or greater pain relief, patient satisfaction with the procedure,
willingness to repeat the procedure if necessary, and measurable increase in ability to perform
everyday activities, based on a point score system we have developed. Patients who did not meet
all these criteria for success were considered failures, determined by disinterested third party
interview.
Results: Psychological data was tested for differences between successful and unsuccessful
treatment outcome and associations between preoperative and postoperative test scores by univariate
and multivariate statistical analyses, in which the dependent variables were test scores which
fell within normal limits. At follow-up, substantial changes were observed between pre- and post-treatment
scores in these patients. Unlike previous retrospective series, significant differences were found
between the successes and failures on these scales. The SCS group's scores returned to normal
limits, while the repeated surgery patients remained outside normal limits.
Conclusions: This suggests the need for careful screening and treatment selections in
FBSS patients, to ensure the overall success (both psychological as well as physiological) of
their surgical or medical treatment.
INSIDE THE MECHANISMS OF MOTOR CORTEX STIMULATION-INDUCED ANALGESIA
B. Laurent, R. Peyron*, L. Garcia-Larrea*, P. Mertens*, N. Costes*, F. Mauguiere*, D. Michel*,
M. Sindou, Neurological Dept, Pain Center, Dept ofNeurosurgery (U400), UPRES (EA 1880) UCLB, CERMEP,
St-Etienne & Lyon, France.
Aim of Investigation: The mechanisms by which Motor Cortex Stimulation (MCS) could mediate
an analgesic effect is studied with Positron Emission Tomography (PET) in a new series of 10 patients.
Methods: 10 patients with central pain were evaluated between 3 and 6 months post-operatively
in the following PET procedure (0-15 labeled water, rest scans, no task, no stimulation). The
MCS had been stopped 24 hours before the PET. Four consecutive scans were first recorded (OFF1).
Then, PET was recorded at 5, 15, 25 and 35 minutes after MCS had been switched on (ON). Then the
MCS was stopped and PET was recorded at 15, 30, 45, 60 and 75 minutes after MCS has been turned
off(OFF2).
Results: MCS (ON vs OFF1) was associated with increased rCBF in the rostral pan of anterior
cingulate contralaterally to the sub-dural electrode. A strong haemodynamic effect was observed
as the MCS was discontinued (OFF2 vs OFF1), including the activation (up to 75 minutes after MCS
discontinuation) of the rostral pan of the contralateral cingulate, the orbito-frontal cortex,
basal ganglia and brainstem. MCS (ON&OFF2 vs OFF1) was associated with decreased blood flow
(suggesting constriction) on the dura immediately below the electrode.
Conclusions: In parallel with long term analgesic effects, MCS demonstrated long lasting
and objective haemodynamic effects. The rostral part of anterior cingulate seems to be the main
region to MCS activity while other subcortical and brainstem structures are likely to be activated
secondanly. These findings suggest that MCS could trigger long-lasting pain control mechanisms
arising from anterior cingulate and descending to brainstem.
Acknowledgments: Supported in part by the Programme Hospitaller de Recherche Climque (PHRC)
1996.
CORONARY ARTERY BYPASS GRAFTING (CABG) VERSUS SPINAL CORD STIMULATION IN SEVERE ANGINA PECTORIS
Clas Mannheimer. Tore Eliasson, Henrik Norrsell*, Hakan Emanuelsson*, Sture Larsson*, Christian
Blomstrand*, Lars-Erik Augustinsson*, Ake Hjalmarson*, Dept of Heart & Lung Diseases, Sahlgren's
Univ Hospital/Sahlgrenska, S-413 45 Gothenburg and Dept of Medicine, Sahlgren's Univ Hospital/Ostra,
S-416 85 Gothenburg, Sweden
Aim of Investigation: Spinal cord stimulation (SCS) has been shown to have anti-anginal
and anti-ischemic effects in patients with refractory angina pectoris. The present study was undertaken
in order to investigate if SCS can be used as an alternative treatment to CAGG in selected patient
groups, i.e. patients with no prognostic benefit from CABG and an increased surgical nsk.
Methods: 104 patients were randomized (SCS 51, CABG 53, 83 m, 21 f, age 63±9). The patients
were assessed with respect to symptoms (anginal attack frequency, consumption of SAN), exercise
capacity, ischemic ECG changes during exercise, rate pressure product, Holter registrations, quality
of life and mortality before and after (7.4±4 months) SCS/CABG.
Results: Both groups had adequate symptom relief (p<0.0001) and there was no difference
between SCS/CABG. The CABG group had an increase in exercise capacity (p<0.05), less ST-segment
depression of maximum (p<0.01) and comparable (p<0,001) work loads and an increase in the
rate-pressure product both at maximum (p<0.001) and comparable (p<0.05) work loads when
compared to SCS. There was no difference in ischemic episodes on 24-hour Holter registration between
the groups. Mortality: 8 deaths (all cardiac) occurred during the follow-up period; CABG 7, SCS
1 (p<0.02). After 3 years the discrepancy between the mortality rate between the groups decreased.
In addition, quality of life data will be presented.
Conclusion: CABG and SCS seem to be equivalent methods in terms of symptom relief in this
group of patients. Effects of ischemia and mortality should be considered in the choice of treatment
method.
IMPROVEMENT OF QUALITY OF LIFE [QOL] MEASURES IN PATIENTS WITH IMPLANTABLE NEU-ROSTIMULATORS
FOR CHRONIC INTRACTABLE PAIN: PRELIMINARY RESULTS
Nagy Mekhail. Armin Aeschbach, Michael Stanton-Hicks, Caroline Androjna, Pain Management Center,
Cleveland Clinic Foundation, 44195, Cleveland, Ohio, USA
Aim of Investigation: To assess the impact of spinal cord or peripheral nerve stimulator
(SCS, PNS) implantation on QOL in patients with chronic intractable pain.
Methods: Sixty patients (61% female, 39% male), ages 21 to 76 years, with SCS/PNS implant
between 1991 and 1998 (average duration post implant of 3.4 years [4 months to 8.1 years]) were
contacted by a disinterested third party and asked to complete a questionnaire.
Results: Primary diagnoses were: Complex Regional Pain Syndrome Type I or II [52%], Failed
Back Surgery Syndrome with residual intractable leg pain [38%], peripheral neuropathic pain [16%],
critical limb ischemia [3%]. 48 SCS and 9 PNS were implanted, and 3 patients had both PNS and
SCS. 73% of patients reported currently using SCS/PNS on a regular basis. Average pain relief
was 52.2% (+/-17.5). 63% reported fewer medication side
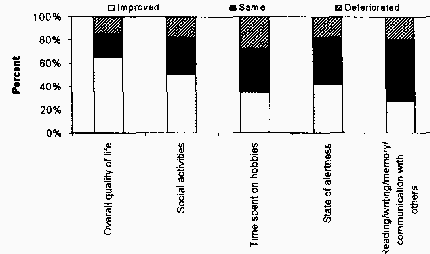
Conclusions: QOL assessment in SCS/PNS for management of chronic, intractable pain is
important given the global impact of this condition on patient's lives. Results of this preliminary
survey reflect trends requiring careful differentiation. While most patients report an overall
improved QOL level, fewer perceive their cognitive functioning and productivity as improved.
Acknowledgement: Supported by a Medtronic Inc. grant.
NEUROLOGICAL AND NEURO PSYCHOLOGICAL FOLLOW UP OF THE ESBY STUDY (ELECTRICAL STIMULATION
VERSUS BYPASS SURGERY)
Henrik Norrsell*. UlfNorrsell*, Christian Blomstrand, Tore Eliasson, Clas Mannheimer, For the
ESBY-study group, Dept of Medicine and Neurology, Sahlgren's Univ Hospital, S-416 85 Gothenburg,
Sweden
Aim of Investigation: In a recently published study Spinal Cord Stimulation (SCS) was
compared to Coronary Artery Bypass Grafting (CABG) in a patient group with no prognostic benefit
from surgery. The aim of this study was to study possible differences between CABG and SCS treatment
on neuro-psychological performance six months after surgery.
Methods: 104 patients were randomised. Before and six months after surgery the patients
were submitted to a thorough examination by an experienced neurologist including a clinical assessment
of neurological and neuro-psychological status. The patients also performed a battery of 17 neuro-psychological
tests under the supervision of a licensed psychologist with neuro-psychological specialisation.
Results: Postoperatively the CABG group performed better on two tests and worse on two
tests. The SCS group performed better on five tests. Patients from the CABG group dominated the
section of patents who were classified as having developed neurasthenia during the study. These
patients were among those that performed worse at follow up tests.
Conclusion: The results of the study indicate that patients subjected to CABG have a higher
risk of becoming neurasthenic than SCS patients. The neurasthenia is associated with an impaired
performance on some neuro-psychological tests.
MOTOR CORTEX STIMULATION IN CENTRAL PAIN. ELECTROPHYSIOLOGICAL STUDY WITH C02 LASER EVOKED
POTENTIALS AND FLEXION NOC1-CEPTIVE REFLEX (RIII).
C. Nuti*. P. Mertens*, L. Garcia-Larrea*, R. Peyron*, P. Con-vers*, F. Maugeiere*, D. Michel*,
B. Laurent, M. Sindou, J. Brunon* (SPON: A. Richard), Hop Bellevue, Bd. Pasteur, 42055 Saint-Etienne
Cedex 2, France
Aim of Study: Motor Cortex Stimulation (MCS) has been shown to induce significant
analgesia in chronic refractory central pain, although mechanisms of pain relief are still to
be understood. In order to evaluate MCS effects on nociceptive pathways, C02 laser evoked-potentials
(LEP) and flexion nociceptive reflex (RIII) were recorded in 7 patients with refractory central
pain and treated with this technique. Pain relief induced by MCS was evaluated with visual analog
scale (VAS).
Methods: LEPs (amplitude and latency of each component) and RIII (surface) were studied
during 3 periods: MSC turned off (at least 12 hours before electrophysiological recordings), MCS
on and MCS off again (at least 30 minutes after MCS interruption). LEPs were obtained after stimulation
of both the painful and the non-affected side. RIII was obtained after stimulation of the painful
side only.
Results: In one patient, after stimulation of the non-affected side, LEPs amplitudes of
the vertex component decreased a lot in the MCS on phase. In the group as a whole, after stimulation
of the non-affected side, LEPs amplitudes tended to decrease under MCS although non-statistically
significant. RIII was not modified in the 3 conditions. Electrophysiological responses did not
correlate with VAS.
Conclusion: LEPs tend to decrease under MCS, even if not significant in this small group
of patients, may help in the understanding of MCS analgesic mechanisms.
192 SPINAL CORD STIMULATION FOR THE RELIEF OF PAIN IN BRACHIAL PLEXOPATHY
Rodriguez. Roberto Fabian. Casagrande Walter*. Division ofNeu-rosurgery. Hospital J. A. Femandez,
Univ, de Buenos Aires. Cer-vino 3356 Capital, Argentina
Aim of Investigation: This study was designed to report: 1] The current follow-up of 6
consecutive painful postganglionic brachial plexopathies, obtained by a disinterested third-party
and to disclose it in visual analogic scale, activities of daily-living, drug-intake and satisfaction
with the procedure. 2] A comparison between the final outcomes of the difference preoperative
pain patterns [evoked, steady and intermittent], and mechanisms [sympathetic dependent [S.D.P.]
and independent] 3] Due to the anatomical task that cervical spine imposes to obtain a consistent
pattern of stimulation-induced paresthesias, a particular technical approach is suggested.
Methods: 6 patients were operated on between 1994 and 1999. The etiology of pain was traumatic
in 5 and postradiotherapy in 1. In all but two sympathetic maintained pain were diagnosed. Allodynia
and steady burning pain were present in all cases, but intermittent pain was dominant in two.
Results: The mean current follow-up period was 16 months, range 2-53; the mean decrease
of the VAS was 6.2 SD 3.6; there was an increase of physical activities of daily living measured
with an ordinal scale; drug intake was reduced and general satisfaction were achieved in all cases.
An analysis of different outcome according to previous pain showed allodynia and S.D.P. as good
prognostic factors.
Conclusions: This therapy should be considered early in painful postganglionic brachial
plexopathies, specially when allodynia and S.D.P. are involved.
193 SPINAL CORD STIMULATION: OUTCOME FOR PATENTS IMPLANTED OVER TEN YEARS
John B Salmon. Phoenix Pain Group, Bethesda Hospital, Claremont, Perth, 6010 Western Australia.
Aim of Investigation: Identification of various outcome measures in 80 patients implanted
with cervical (n29) or lumbar (n33) spinal cord stimulation (SCS) systems over the last ten years.
Average duration of SCS 3.76 years, stimulation on average 7.8hrs/day. Methods: Total of 80 patients
implanted by author. 90% of these were followed up. 63 patients completed questionnaires comprising
a specific SCS outcome measure on a 6 point Likert scale assessing 6 dimensions - pain reduction,
medication reduction, increase in function, return to work, sense of control and capacity to enjoy
life. Also the BDI, SF36, PSEQ and CSQ questionnaires were administered. Current occupational
status, medication consumption and additional treatment consumption were also identified.
Results: 84% have good pain reduction. 48% have ceased routine analgesic consumption,
26% remain on oral opiates. Light to moderate activity/function increased significantly in 70%.
38% of "work eligible" have returned to work, 51% of work eligible working. 66% had
no further treatment post-SCS implant. 85% no longer significantly depressed (BDI < 18).
Conclusions: Cervical and lumbar SCS treatment may provide positive long term benefit
as judged by a number of basic measures. This result also infers a positive cost benefit outcome.
SCS was particularly effective for cervico-brachial pain syndromes and should be utilised relatively
early in patients refractory to other treatment.
TREATMENT OF CHRONIC NEUROPATH1C PAIN BY PRECENTRAL CORTICAL STIMULATION
Sindou M.. Mertens P.*, Peyron R.*, Guenot M.*, Nuti C.*, Garcia-Larrea L., Mauguiere F.*, Laurent
B., Federative Inst for Neu-rosciences - 69003 Lyon, France
Aim of Investigation: Precentral cortex stimulation has been introduced by TSUBOKAWA in
1991 for post stroke pain and by MEYERSON in 1993 for trigeminal neuropathic pain. The purpose
of this present paper was to report the clinical results of cortical stimulation in a prospective
study of 20 patients suffering from intractable central neuropathic pain.
Materials: Since 1992, 20 patients were explorated (10m, lOf, 52y on a.): 12 with post
stroke lesion, 3 with spinal cord lesion, 4 with brachial plexus avulsion. In all patients the
VAS for pain was higher than 8. In 13 cases one, and in 7 cases two quadripolar elec-trode(s)
were implanted extradurally on the precentral gyrus in respect to somatotopic representation of
the controlateral painful area. The parameters used for chronic stimulation were: rate 20 to 60
Hz, intensity under threshold of motor responses, pulse width 60 ms, cyclic period of stimulation:
30 min on -60 min off.
Results: At the later following assessment (21 months on a.), 5 patients experienced more
than 80% pain relief, 8 patients between 80% to 30% and 7 patients less than 30% (=failure). Success
rate vaned from 100% for myelopathic pain, to 66% for post stroke pain and 50% for brachial plexus
avulsion.
Conclusion: Provided further studies confirm these preliminary results, as well as those
from the literature, precentral cortex stimulation will probably take place in the near future
within the armamentarium for chronic pain treatment.
FUNCTIONAL IMPROVEMENT IN PATIENTS WITH IMPLANTABLE NEUROSTIMULATORS (IN) FOR CHRONIC INTRACTABLE
PAIN: PRELIMINARY RESULTS
Michael Stanton-Hicks. Armin Aeschbach, Nagy Mekhail, Pain Management Center, Cleveland Clinic
Foundation, 44195, Cleveland, Ohio, USA
Aim of Investigation: To assess changes in Activities of Daily Living (ADL) after the
implantation of a Spinal Cord or Peripheral Nerve Stimulator (SCS, PNS) for chronic intractable
pain.
Methods: All patients implanted with a SCS or PNS at the Center between 1991 and 1998
were contacted by telephone by a disinterested third party and were asked to fill out a standardized
questionnaire.
Results: The results of the first 60 patients of this survey are summarized. Ages at follow-up
were 21 to 76 years. Average duration since implant was 3.4 years (4 months to 8.1 years), 61%
were women. Primary diagnosis was in 51% Complex Regional Pain Syndrome Type I or II, 38% Low
back and leg pain, 16% peripheral neuropathic pain and 3% painful limb ischemia. 48 SCS and 9
PNS were implanted, 3 patients had both PNS and SCS. 73% were still using their implant.
Self-Assessment: Function
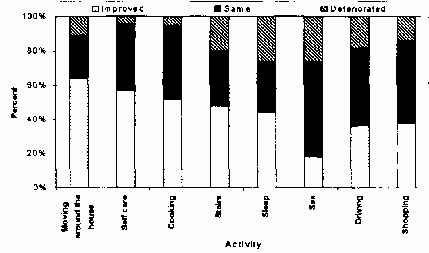
Conclusions: Change in everyday activities is an accepted outcome measure. So far little
is available in the literature on the performance of individual tasks, however. It appears from
this survey that a majority of patients with in's improve in most ADL's concerning the household.
A smaller percentage improves with more strenuous functions such as heavy yard work, driving and
shopping.
Acknowledgement: Supported by a Medtronic Inc. grant.
PERIPHERAL NEUROSTIMULATION TO CONTROL INTRACTABLE OCCIPITAL NEURALGIA
Richard L. Weiner. Kenneth M. Alo1, Michelle L. Fuller, Dallas Neurosurgical Associates,
Presbyterian Hospital of Dallas, 8230 Walnut Hill Lane, Suite 220, Dallas, TX 75231, USA Email:
rlwl@ix.netcom.com, 'Pain and Health Management Center, Houston, TX
Aim of Investigation: To evaluate the effectiveness of a new subcutaneous peripheral nerve
stimulation technique for the treatment of refractory occipital neuralgia.
Methods: Beginning in late 1992 a new surgical technique involving subcutaneous insertion
of single or dual percutaneous peripheral nerve Stimulator electrodes was developed for treatment
of intractable occipital neuralgia by the senior author The implants at the level ofCl utilized
a variety ofquadripolar and octapolar lead systems (Medtronic Inc, and Quest-ANS Inc.). We have
further refined the surgical technique and have collected follow-up data on 37 implants in 33
patients over a six-year interval.
Results: Patient outcome rates to date: excellent and persistent relief of pain in 18
(55%) patients, Good relief of pain in 9 (27%) patients, fair relief of pain in 6 (15%) patients.
Two patients were subsequently explanted (one good, one fair) and two patients are deceased (both
fair).
Conclusions: Subcutaneous application of peripheral nerve stimulation techniques at the
level ofCl appears to be reasonably effective in controlling otherwise intractable occipital neuralgia
type headache pain and should be considered as a treatment alternative to more aggressive surgical
interventions.
9th WORLD CONGRESS ON PAIN, 1999, Vienna, Austria, p.57 - 61
|

